Table of Contents
DATE: Dec. 8, 2014
LOCATION: Chandler 260
SUBJECT: Pouring of tin
Once we made sure that the mold was completely dried, we went ahead and prepared to pour the tin with Joel. Michelle (with a little too much glee) melted the tin and poured it into the mold. once we had left it to cool long enough, we removed the metal from the mold. the result was quite stunning; the metal had poured beautifully and picked up all the detail from the mold impression, and also had a beautiful antique/gold tarnished finish. Professor Smith told us that the gold tinge was most likely due to the salt in the binder solution, which leads us to wonder whether or not the sandever would have had the same sort of effect on the metal as well; mostly likely so, since sandever is supposed to be saline, and a bitter salt. Also, the sand itself cleanly separated from the metal and was left intact, enough so that if one wanted to mold again, it could easily be done.
 |
| detailed shot of the metal and the original plaster cast |
 |
| mold, plaster cast and metal cast all together |
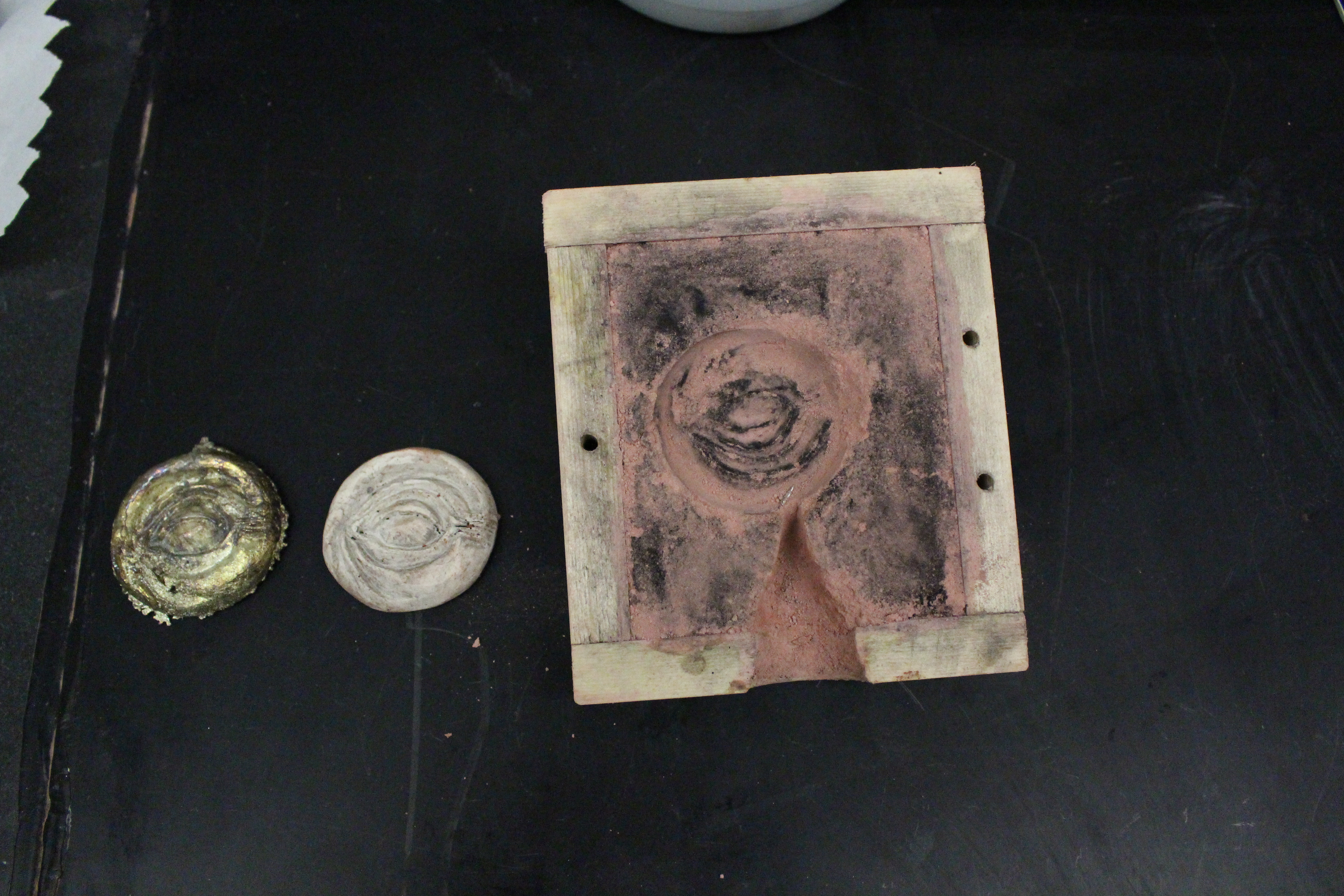 |
| another view of the metal, plaster cast and mold |
Conclusion: the experiment seems to have been successful, since what we were looking for was a clean impression and a clean metal result, with an intact mold left over after. we are still a little curious as to why it crumbled on the bottom half, though it could be because of the carving (it did carve inward and the sand could have just stuck in those crevices), but we are not sure. also, maybe with this binder, tin should be avoided? would other metals be tarnished by the salt/sandever as well?
NAME: Michelle Lee, Diana Mellon, Yijun Wang
DATE: Dec. 5, 2014
LOCATION: Chandler 260
SUBJECT: Prep & logistics
Sand
We ground and sifted the sand contents (ground old molds) from 1.5 of the wooden molds we used in Ubaldo Vitali's studio.
Oil of tartar
Koon Chun Potassium Carbonate & Sodium Bicarbonate solution, purchased at supermarket in Chinatown
Test 1
- 1 T pulverized rock salt in 60 mL Potassium Carbonate & Sodium Bicarbonate solution
- Stir with wooden chopstick, add to sand. It's still too dry...
- Add: 1 T pulverized rock salt dissolved in 60 mL Potassium Carbonate & Sodium Bicarbonate solution
- Add: 1 T pulverized rock salt dissolved in 60 mL Potassium Carbonate & Sodium Bicarbonate solution
- Placed plaster eye medal, dusted in charcoal, flat side down on stone counter surface, surrounded by inside first half of wooden box mold frame
- Packing the sand/rock salt/oil of tartar combo into this half of the wooden box mold frame
- Everything about the packing process feels easier this time! Is it because we've done this before, when we packed our molds for Ubaldo Vitali's studio, or is it because this binder is easier to work with?
- Continuing... we dust the entire surface with charcoal and then add the second half of the frame and continue packing
- We open the mold--it looks great, comes apart cleanly!
- We carve a channel for the metal, but when we try to remove the medal, small chunks of sand come with it, leaving an imperfect impression
- Why did this happen? Should we have waited to let our molds dry before removing the pattern? Should we have dusted more charcoal onto it? Should the sand have been more wet?
Test 2
- We returned the sand to the bowl and mixed in one more beaker of solution (1 T pulverized rock salt in 60 mL Potassium Carbonate & Sodium Bicarbonate solution)
- We dusted the pattern with charcoal, generously this time
- Packed the sand into one half of the mold
- The sand remaining in the bowl feels a bit drier, so we add 20 mL of the above rock salt/Potassium carbonate/Sodium bicarbonate solution
- pack the remaining side, then go ahead and open the mold.
- impression is cleaner than the last, but still a little crumbled on the bottom half. after much deliberation, we decide to just cast the metal with this second test mold, since the impression is otherwise very clean.
- leave the mold to dry over the weekend, to be poured with tin after it is completely dried.
NAME: Michelle Lee, Diana Mellon, Yijun Wang
DATE: Dec. 3, 2014
LOCATION: W. 113th St & Chinatown
SUBJECT: Oil of tartar
- Trying to light Cream of Tartar (Potassium bitartrate) on fire to burn it to Potassium carbonate
- Put a small amount of Cream of Tartar into a Pyrex dish in the bathtub, tried to ignite it with a lighter
- The top particles turn black after we hold the lit flame over it for a while, but this doesn't seem to be the result we're looking for. The Cream of Tartar doesn't catch and sustain its own flame.
- We try putting an even smaller about of Cream of Tartar in a Pyrex dish with alcohol. The alcohol burns, but the Cream of Tartar responds as above. Even less of it turns black.
- We discover through the internet that Potassium carbonate is an ingredient in some ramen recipes! We find a supermarket where we buy 500 mL of Koon Chun Potassium Carbonate & Sodium Bicarbonate solution
Recipe
Magistry [in French, has the same title as p084v, “Eau Magistra”]Dissolve rock salt or sandever that you have pulverized finely and placed on a marble slab in a pot. It will dry out while you reheat the mold, and will provide it with a binding to enable several castings. Try moistening it with tartar oil.
[italics in manuscript!]
Eau magistra
Fais dissouldre le sel gemme ou selsuin de verre subtilem{ent}
pulverise & mis sur un mabre dans une cave & il se desseichera
en recuisant le moule & luy donnera une liaison pour soubstenir
plusieurs gects Essaye de humecter avecq huile de tartre
Recipes in MS
“VOCABULARY” doc on shared drive:
- “Eau magistra, also short magistra, magistre = “magistry”
A liquid used to bind the sand in sand molding. On 84v, 87v, there is a recipe for it. See also: Orme”- “Orme. (m.) = “elm”.
The roots are always boiled in wine (“or better yet, vinegar”, it says on 087v) in La boutique; see 087v, which explains that elm roots boiled in wine produce Magistra; for occurrences (with or without being identified as magistra), see also 86r, 72r, 69v; if you encounter this recipe, please add your occurrence here”
- “Orme. (m.) = “elm”.
p084v Eau Magistra
Some people think that salt water is not good, because the salt releases gas when heated and as a result causes bubbles. [In this case], there is only wine boiled with elm root.
p087v Magistry
Founders harvest the roots of a young elm when it is sappy, and boil it in wine, or better yet vinegar. They prepare a year’s worth of it and store it in a cask.
p154r Metal file dust
It does not melt by itself if it is not helped with some portion of similar metal melted to assemble it and bathe it, as it is more burnt than melted. File dust from tin and lead are made with tallow, [file dust] from gold with saltpeter, [file dust] from silver with sandever.
Glass Gall
The art of glass, wherein are shown the wayes to make and colour glass, pastes, enamels, lakes, and other curiosities by Antonio Neri (d. 1614)
Written in Italian. Translated into English, with some observations on the author [by C.M., i.e. Christopher Merret] Whereunto is added an account of the glass drops made by the Royal society meeting at Gresham College. London, Printed by A.W. for O. Pulleyn, 1662. Original in Columbia's rare book collection.
Pages 276-77 on sandever:


The Culture and Technology of Glass in Renaissance Venice, William Patrick McCray, diss. University of Arizona, 1996:
See especially "The Science and Technology of Cristallo Production" pp. 392-
"As a result, two additional processing steps were used to get rid of excess
chlorides and sulfides in the glass melt. One step was to skim the top surface of the
glass melt and remove any impurities. There would typically be a floating molten
layer formed by unreacted and undissolved chlorides and sulphates which did not
mix with the molten glass (Verita, 1991:60). The Venetians used a long-handled tool
called a partigola to skim the surface of the glass." (McCray diss., 399)
"Neri elaborates further on it in his recipe called "To make crystal in full perfection, the way I always practice"
(Chapter 9, book 1):
To make a fair crystal, when it is well-melted take it from the pot and cast it
into great earthen pans or clean bowls fiill of clean water. To this end the
water may take from it a sort of salt called sandever which hurts the crystal
and makes it obscure and cloudy...then put it again into a clean pot and cast
it into water which is to be repeated as often as needed." (McCray diss., 400)
"The product that results from pouring the glass into water and removing the
chloride and sulphate impurities is called cotizzo (Moretti, 1983:182). This word
stems from the Italian for cooking - cottura. The English translation of Neri's book,
prepared by Merrit in 1662, notes that the sandever was an article of commerce in 01
the 17th century. This word comes from the France term suin de verve meaning "the
fat of the glass". This was exported from England to France and used as a flux for
metal working, as a weed killer, and as a food preservative (Turner, 1956b:295)"
(McCray diss., 400-401. See below for relevant passage from Turner.)
mccray venetian glass diss.pdf
azu_td_9720571_sip1_... - Home - The University of ...
The quotes above are from W. Patrick McCray's dissertation, but see also his book on the history of Venetian glassmaking: The Fragile Craft (thanks to Walt Lieberman of the Museum of Glass in Tacoma, WA for leading us to W. P. McCray!)
W. E. S. Turner, "Studies in Ancient Glasses and Glassmaking Processes. Part V. Raw Materials and Melting Processes," Journal of the Society of Glass Technology, vol 40, 1956, entire chapter in PDF below. Here pp. 295-6:

Turner Raw Materials and Melting Processes.PDF
Ian Hankey, video on glassmaking & embodied knowledge:
http://www.culturalhistoriesofthematerialworld.com/books/ways-of-making-and-knowing-the-material-culture-of-empirical-knowledge/multimedia/
Comment from Adham Azab in 84r “tc” doc:
suis de verre
Suif de verre : http://books.google.com/books?id=daA-AAAAYAAJ&lpg=PA104&ots=Vrx806AOcD&dq=%22suif%20de%20verre%22&pg=PA104#v=onepage&q=%22suif%20de%20verre%22&f=false
The filth or greasiness of a sheep's wool before it is washed, or suif de verre, sandiver
This said, Merriam Webster leads us to the MF "saïn de voirre", and indeed, the word in our manuscript is clearly suin(t), which also has to do with fattiness. Perhaps we need look no further than Cotgrave: "Suin de verre. Sandever; the fattie substance floating on glasse when it is red-hot in the furnace, and which being cold is as hard as stone, yet brittle, and easily broken."
makingandknowingproject://
Dufief, Dictionnaire nouveau et universel (1810), Google Books.
However this word is already in the vocabulary; comment moved to tl
“VOCABULARY” doc (on shared drive):
- Suin de verre (m.) = “glass-gall”, also “sandever” (FP)
Randle Cotgrave, Dictionarie of the French and English Tongues (London 1611), under the entry for salt:
- “suin de verre” = sandever:
| Screen Shot 2014-11-05 at 6.25.22 PM.png |
Sandiver or Glass-Gall, Brick, Pottery and Glass Journal (1879-1880); Jul 1, 1879; 6, 7; American Periodicals, p. 77:

glass gall.pdf
The art of glass, showing how to make all sorts of glass, crystal, & enamel ... Illustrated with proper sculptures. Written originally in French, by Mr. H. Blancourt, & now first tr. into English ...
Author: Haudicquer de Blancourt, Jean, b. ca. 1650
Language: English
Published: London, Printed for D. Brown. T. Bennett, [etc., etc.]. 1699.
Physical: 355 p. plates. 20 cm.
FULL TEXT in HathiTrust via Clio
Chapter X. "The way to make a Salt of several Vegetables, which will produce a Crystal of a wonderful Fineness”
Chapter XI. "To make a very fair Crystal of Salt of Lime"
Chapter XIII. "The way to make very fine and perfect Crystal"
de blancourt 58.pdf
E-mail from Glen B. Cook, Senior Research Associate (future Chief Scientist), Corning Museum of Glass, 11/12/14:
Dear Ms. Mellon –
My name is Glen Cook, and I am a research scientist with Corning Incorporated, and soon to be (starting in Jan 2015) Chief Scientist at the Corning Museum of Glass. Thank you for contacting the museum about your fascinating research project.
You are correct in your understanding that glass gall/sandever is rare. In modern glass production the raw materials that are charged into the furnace (“the batch”) are very carefully controlled by strict quality control measures. Ingredients that would produce gall (e.g., chlorides and sulfates of sodium or potassium) are scrupulously excluded if possible; and if they are charged (as fining agents or colorants), the actual amounts are at levels far below levels that would lead to gall.
The exception is for operations that try to make usable glass by charging a combination of known-quality glass batch or cullet with various hazardous waste streams, such as demolition wastes containing asbestos. These less-controlled ingredients can contain significant amounts of materials with limited solubility in the glass melt, like sulfates and chlorides, that then float or sink (depending on their densities when molten) to form a top scum layer, or bottom sludge. Special provisions must be made in the design of the melting furnaces that do this kind of work, to enable the skimming or draining of the gall. An interesting side note, that you may be aware of, is the use of the term “gall” for this material likely derives from the fact that the fused, cooled sulfate-containing salt would be bitter to the taste – “galling.”
I regret that I am not familiar with any manufactures of this type of glass material, however, that would have gall available. I am unsure, actually, if the glass itself would need to be treated as hazardous waste. Perhaps the information above can be a lead to help you find someone in your area who is doing hazardous waste vitrification.
Another option that you might consider is to contact glass artisans who specialize in historical recreation of historical glass melting techniques.
I hope this information is useful. Best wishes on the success of your project. I ask, if you would please, that you keep me apprised of your work as you move forward.
Kind Regards,
Glen Cook, Ph.D.
Senior Research Associate
Corning Incorporated
POTT, Johann Heinrich, Untersuchung der Natur und Eigenschaften der Glasgalle
pott_galle.pdf
Oil of Tartar
After discussion with Joel Klein and further research:
- Potassium Bitartrate = cream of tartar (melting point: 446 F)
- Potassium Carbonate = salt of tartar, pearl ash, (oil of tartar when water is added)
- Wikipedia's Potassium Bitartrate page(italics added):
“Potassium bitartrate is NIST's primary reference standard for a pH buffer. Using an excess of the salt in water, a saturated solution is created with a pH of 3.557 at 25 °C (77 °F). Upon dissolution in acid, potassium bitartrate will dissociate into acid tartrate, tartrate, and potassium ions. Thus, a saturated solution creates a buffer with standard pH. Before use as a standard, it is recommended that the solution be filtered or decanted between 22 °C (72 °F) and 28 °C (82 °F).[6]Potassium carbonate can be made by igniting cream of tartar producing "pearl ash". This process is now obsolete but produced a higher quality (reasonable purity) than "potash" extracted from wood or other plant ashes.”
“VOCABULARY” doc (on shared drive):
- Tartre (m.) = “tartar”; sel de tartre = “salt of tartar”
Pigment glossary (on shared drive):
Sediment of yeast and other matter containing potassium hydrogen tartrate of wine casks and on corks. Once purified, this is white cream of tartar, used in cooking. Calcining the potassium hydrogen tartrate (cream of tartar) gives the alkali potassium carbonate; lume de feza is potassium carbonate obtained this way. Charred wine lees, with the alkali removed by washing, give Frankfurt black (q.v.). COOH), also seen as red crystals on the inside
Randle Cotgrave, Dictionarie of the French and English Tongues (London 1611), under the entry for salt:
- “huile de tartre” = tartar oil:
| Screen Shot 2014-11-05 at 6.28.01 PM.png |
British Eighteenth-Century Chemical Terms - Part 2
- Oil of Tartar: "Concentrated potassium carbonate solution (K2CO3)."
- Oil of Tartar per Deliquium: "Potassuim carbonate, which is hydroscopic, dissolved in the water which its extracts from the air.”
- From Alchemical Glossary entry for "Per deliquium," Chymistry of Isaac Newton website: "Literally, 'by dissolution,' referring to hygroscopic materials (e.g., salt of tartar) that are allowed to dissolve in the humidity of the open air."
Vocabluary after Palissy, 1902 (on shared drive):
| Screen Shot 2014-11-05 at 6.08.04 PM.png |
Lemery, Cours de la Chymie, Paris, 1675, p. 442, translation from here:
“Break the horn you have used for the distillation of tartar and get hold of the black mass you will find inside, calcined it at coal till white, then throw it into a big volume of hot water and lixiviate, filter it and pour it in a glass, or earthen, vessel and let evaporate the humidity at sand heat. It will remain a white salt, called Salt Alkali of Tartar.
This salt is aperitif and is used to extract the Vegetal Tincture. The dosage is from ten to thirty grains. If you will expose this salt for a few days in a glass platter in a cellar, it will reduce in a liquor called “Oil of Tartar by failure”. It is used as a cosmetic and against cancer ( note the order of importance).
Observations.
By means of these operations we have extract all we can from Tartar, but those who are not interested neither in Oil nor in Spirit and want just to extract the Salt can employ the untreated, or raw, Tartar and having wrapped it up in a paper, calcine it in red hot coal till it will be reduced in a white mass, after that they extract the salt by lixiviation, as mentioned above.
The liquor, or oil, made by failure, is just a Salt of Tartar dissolved in a cellar humidity. This Salt has to be melted only in well filtered rain water.“
Idea Of a table booke, Portsmouth Collection Add. MS. 3975, Cambridge University Library, Chymistry of Isaac Newton:
"The first complete edition of what is perhaps Newton's most important laboratory notebook. It is a bound volume of over one hundred-seventy folios. It contains recorded dates ranging from 1669 to 1693, but parts of the notebook, such as the optical section on ff. 2r–12v, may have been composed earlier. The notebook is remarkable for the way in which it reveals how several of Newton's interests were related, particularly his chymistry and optics."
- Various forms of tartar mentioned, including "Oyle of Tartar" (see link above)
- Some forms have medicinal properties
- Symbol for Tartar shown in symbol guide:

Rock Salt
“VOCABULARY” doc (on shared drive):
- Sal gemme = “rock salt”
Randle Cotgrave, Dictionarie of the French and English Tongues (London 1611), under the entry for salt:
- “Sel gemme’. A transparent, and shining minerall salt, gotten in Calabria, and Capadocia.”
- “sal gemme’” = rock salt:
| Screen Shot 2014-11-05 at 6.21.00 PM.png |